New study on human egg formation paves way for artificial ovaries
Artificial ovaries are on the horizon after scientists created the first cellular-level map of human egg formation.
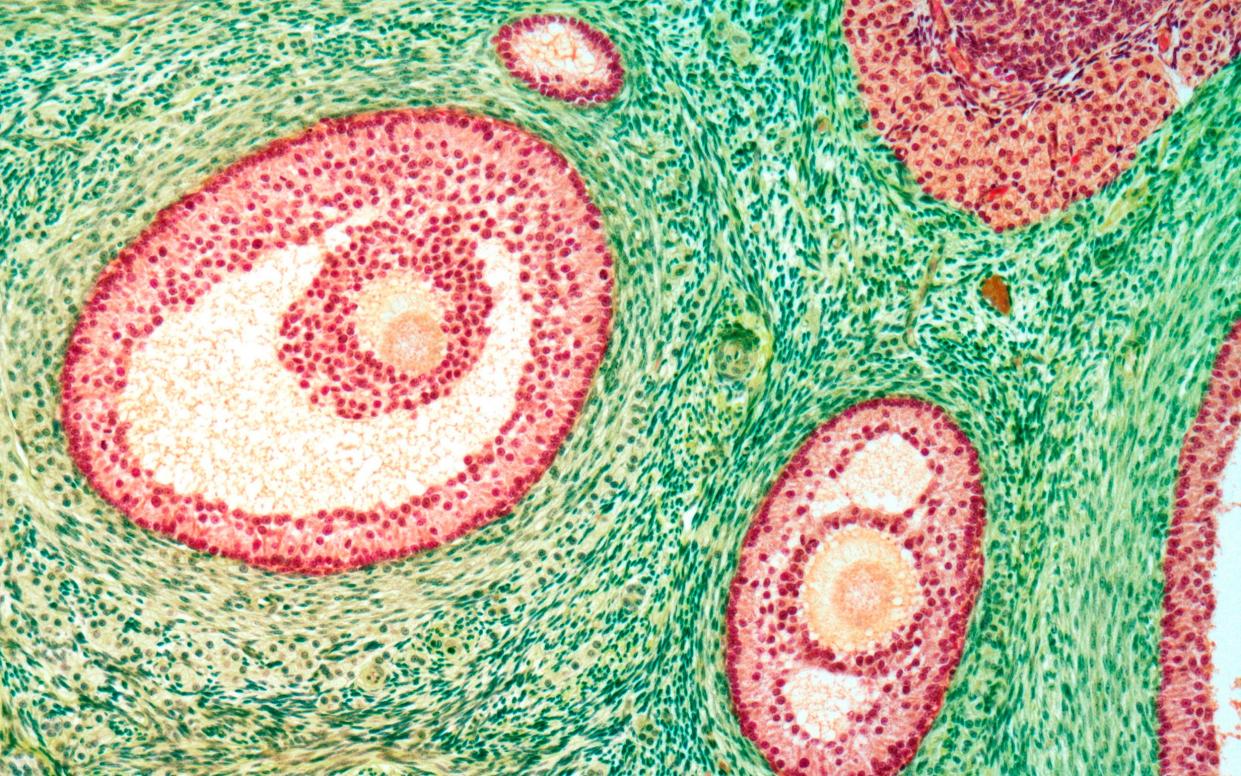
The University of Michigan used five donated ovaries to carry out state-of-the-art cell and genetic mapping to discover what is needed for a follicle to mature and produce an egg.
Researchers said unpicking the process paved the way for creating artificial ovaries in the lab using tissues that had been frozen and stored, such as by cancer patients before toxic medical treatments.
Currently, surgeons can implant previously frozen ovarian tissue to temporarily restore hormone and egg production, but fertility does not last long because so few follicles survive implantation.
If scientists can create more follicles that mature and produce eggs, it would extend the fertility window for cancer patients and offer new hope for infertile women.
“Now that we know which genes are expressed in the oocytes, we can test whether affecting these genes could result in creating a functional follicle,” said Dr Ariella Shikanov, the associate professor of biomedical engineering at the university.
“This can be used to create an artificial ovary that could eventually be transplanted back into the body.”
Ovarian follicles are small sacs filled with fluid that can produce eggs, and each woman begins puberty with around 300,000 to 400,000.
The majority of the follicles remain dormant with only a few getting activated and migrating into the ovary, to a region known as the growing pool, where an even smaller number goes on to produce mature eggs that are released into the fallopian tubes.
The new atlas reveals the factors that enable a follicle to mature, as most follicles wither away without releasing hormones or an egg.
Working towards magic
Researchers were able to identify what genes are being expressed at a single-cell level within a tissue of the ovarian follicles, and are hopeful they will be able to guide this development process, encouraging more follicles to activate and produce eggs.
“We’re not talking about utilising a surrogate mother, or artificial insemination,” said Jun Z Li, the associate chair of UMich’s Department of Computational Medicine and Bioinformatics and co-corresponding author of the study.
“The magic we’re working toward is being able to trigger an immature cell into maturity, but without knowing which molecules drive that process, we’re blind.”
The team used a new technology called spatial transcriptomics, to track all of the gene activity—and where it occurs—in tissue samples.
“This was the first time where we could target ovarian follicles and oocytes and perform a transcription analysis, which enables us to see which genes are active,” added Dr Shikanov.
“The majority of ovarian follicles, already present at birth, never enter the growing pool and eventually self-destruct.
“This new data allows us to start building our understanding of what makes a good egg – what determines which follicle is going to grow, ovulate, be fertilised and become a baby.”
The work is part of the Human Cell Atlas project which aims to create maps of all cells of the body, to understand how they function and what goes wrong during disease.
The new research was published in the journal Science Advances.


