One dead in bacteria outbreak linked to eye drops from India
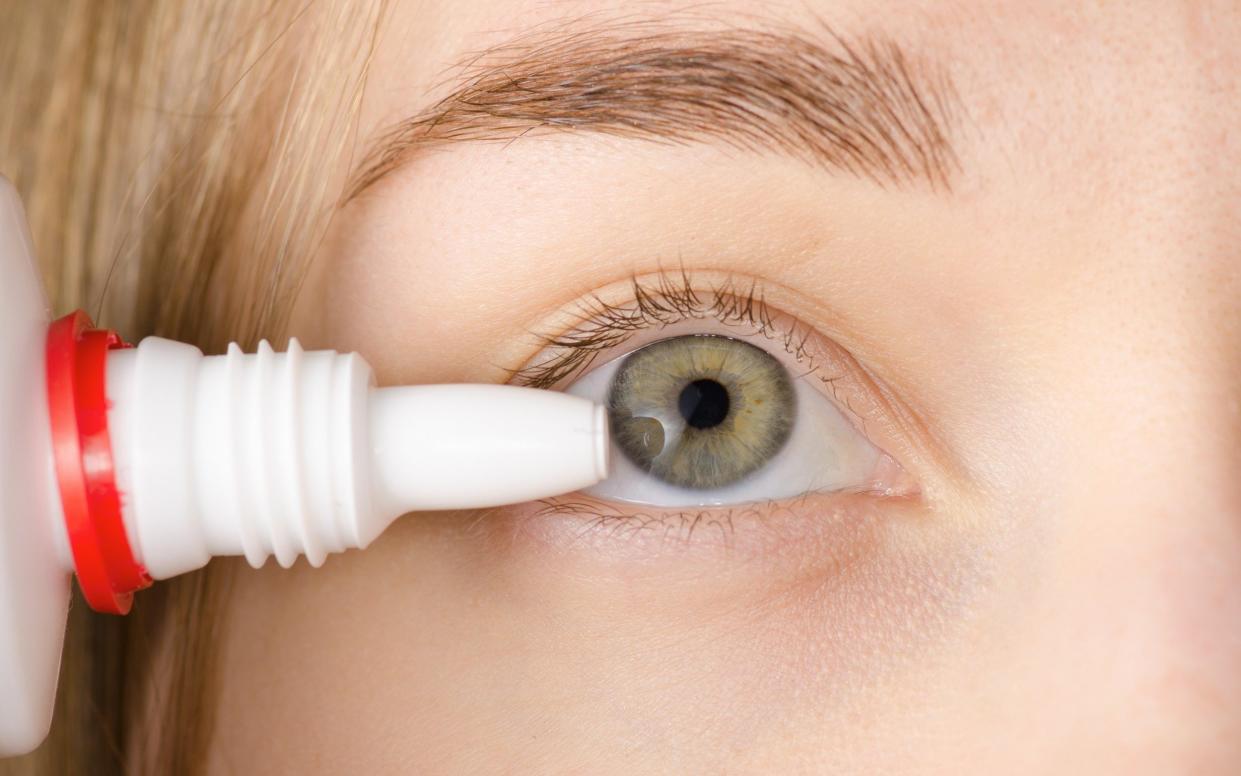
One person has died and dozens more have been infected with antibiotic-resistant bacteria in an outbreak that has been linked to eye drops from India.
Health officials said at least 52 people had been infected by the burkholderia cepacia bacteria, which has been traced back to three separate lubricating eye drops.
The bacteria is known as a superbug because it is resistant to antibiotics, which makes it much more difficult to treat and potentially fatal in people with weakened immune systems.
One person is confirmed to have died from the outbreak, but no details have been made public.
There have been 52 confirmed cases and six probable cases, according to the UK Health Security Agency (UKHSA). The youngest victim was a baby and the eldest a 91-year-old.
In 25 cases there were “clinically significant infections”, according to the UKHSA, while 11 people suffered eye infections, which included ulcers on their eyeballs, conjunctivitis or serious “deep tissue infections”.
Nine patients developed respiratory infections and four more had blood poisoning.
The health authority issued a product recall last November for the three products manufactured by Indiana Ophthalmics, which is when most cases are understood to have occurred.
Officials now believe the outbreak is over, although cases continued to emerge until February this year.
It is thought various batches of AaCarb, Aacomer and Puroptics branded carbomer eye gels were affected.
The products are usually given to patients suffering from dry eyes, but they can also be bought online for as little as £4.50.
The vast majority of patients were being treated in hospital for a separate issue when they were given the eyedrops by staff, who were unaware the products were contaminated.
This led the UKHSA to issue a national patient safety alert in December, advising all medics in the NHS to avoid using carbomer eye gels on high-risk patients, such as those going through chemotherapy.
This advice was withdrawn in an update on Tuesday as the health agency said the outbreak had slowed, but added that the situation would still be monitored.
The Medicines and Healthcare products Regulatory Agency says it has now received “sufficient assurance from manufacturers and suppliers to conclude that products available on the UK market are safe to use and free of contamination”.
UKHSA said it would “continue to follow up new cases and keep vigilance for emergent clusters of Burkholderia cepacia complex.”


